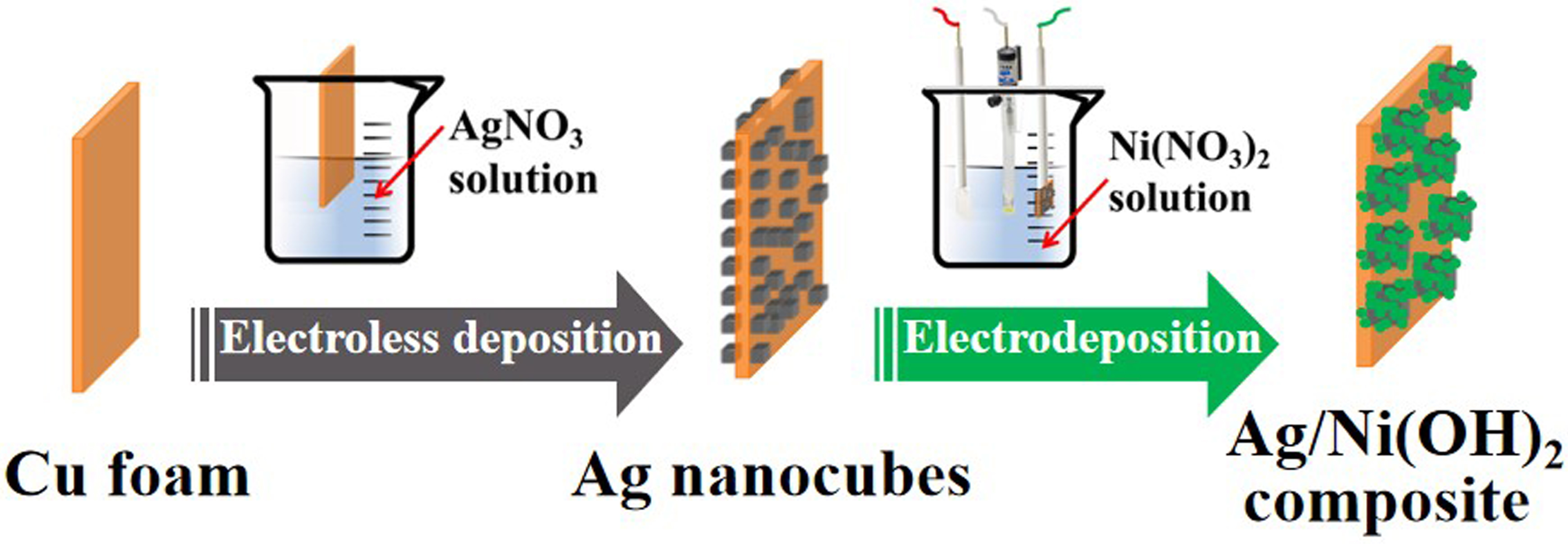
Metals | Free Full-Text | In Situ Construction of Ag/Ni(OH)2 Composite Electrode by Combining Electroless Deposition Technology with Electrodeposition

SOLVED: Nickel is a more active metal than silver; therefore, nickel will replace silver ions in a silver nitrate solution to form Nickel (II) nitrate and silver metal. How many grams of

Oxidation of Aniline with Silver Nitrate Accelerated by p-Phenylenediamine: A New Route to Conducting Composites | Macromolecules

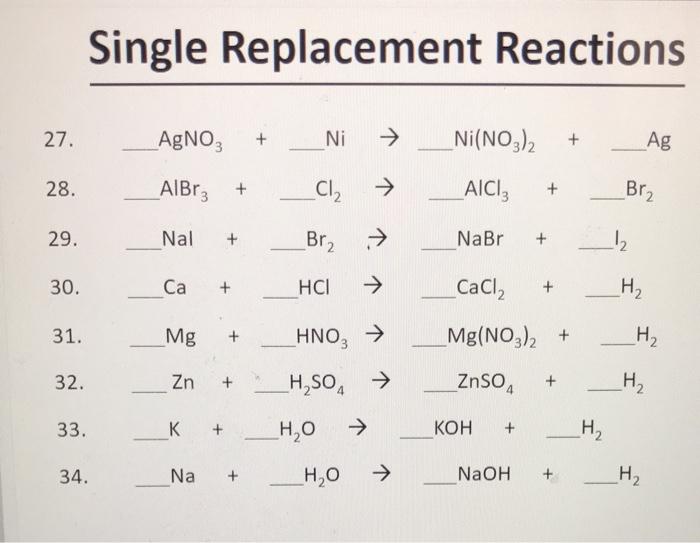
balance the following chemical equations and identify the reactants and products. Zn + AgNO3 ------Zn(NO3)2 - Brainly.in

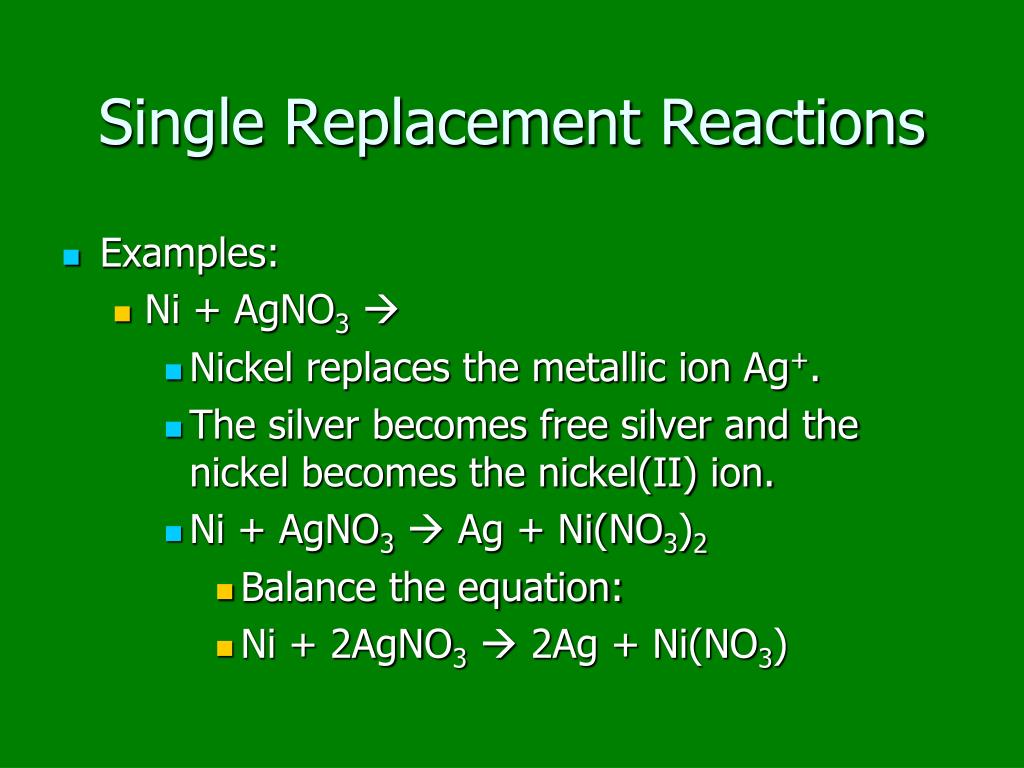
OneClass: 9. Based on the equations below, which metal is the most active? Pb(NO3)2 (aq) + Ni (s) â†'...

Trimetallic CuO/Ag/NiO supported with silica nanoparticles based composite materials for green hydrogen production | Scientific Reports




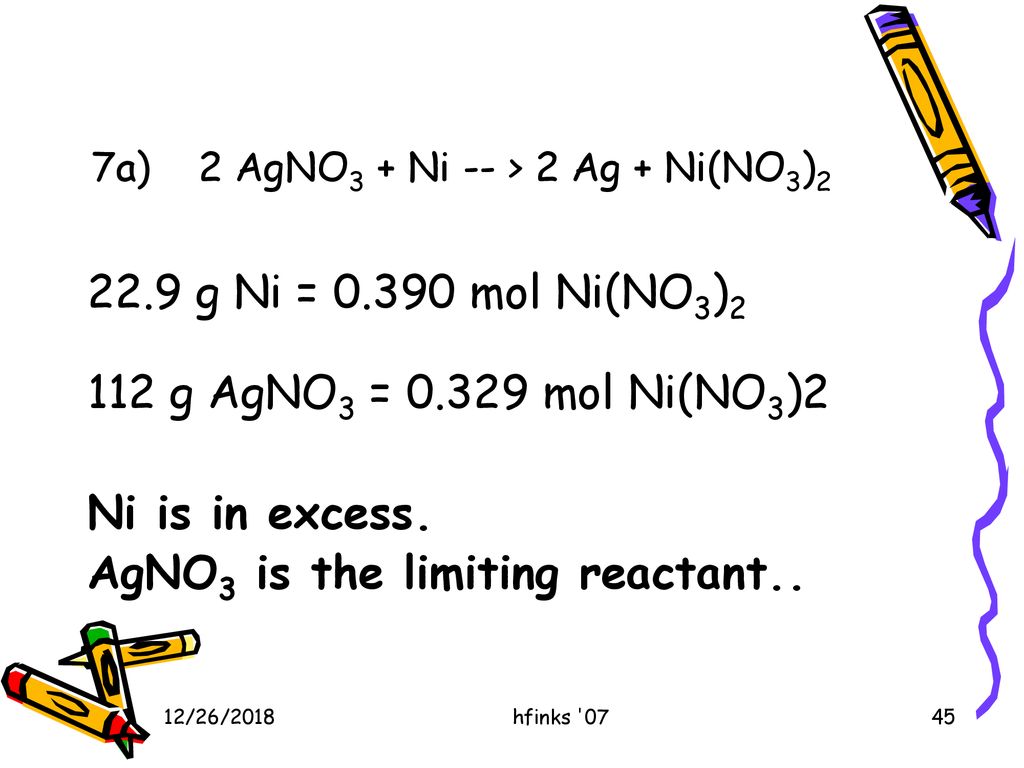


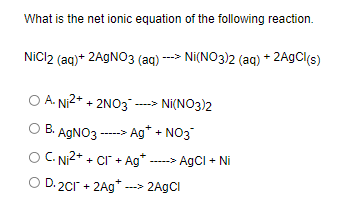


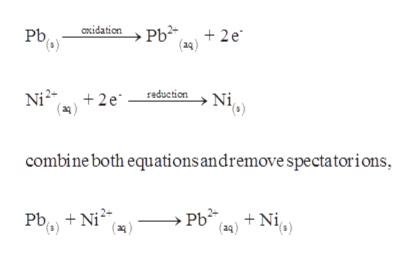

![Kannada] A strip of nickel metal is placed in a 1molar solution of Ni Kannada] A strip of nickel metal is placed in a 1molar solution of Ni](https://static.doubtnut.com/ss/web-overlay-thumb/7678142.webp)
