2. All atoms, except the | Download Scientific Diagram Representation of the unit cell of [Ni(H2O)6](NO3)2. All atoms, except the | Download Scientific Diagram](https://www.researchgate.net/publication/269400063/figure/fig5/AS:667921089052683@1536256205687/Representation-of-the-unit-cell-of-NiH2O6NO32-All-atoms-except-the.jpg)
Representation of the unit cell of [Ni(H2O)6](NO3)2. All atoms, except the | Download Scientific Diagram
✓ Solved: Describe the distribution of d electrons in [Ni(H2O)6]^2+, using crystal field theory. How...
![Draw the CFT diagram for [Ni(H2O)6]2+ (NO LINKS STRICTLY)!!! - Chemistry - Coordination Compounds - 11664449 | Meritnation.com Draw the CFT diagram for [Ni(H2O)6]2+ (NO LINKS STRICTLY)!!! - Chemistry - Coordination Compounds - 11664449 | Meritnation.com](https://s3mn.mnimgs.com/img/shared/content_ck_images/ck_599e6db7e641a.png)
Draw the CFT diagram for [Ni(H2O)6]2+ (NO LINKS STRICTLY)!!! - Chemistry - Coordination Compounds - 11664449 | Meritnation.com
![SOLVED: The compound [Ni(H2O)6]Cl2 is paramagnetic. Determine the oxidation number of nickel in this compound, the most likely geometry of the coordination around the nickel, and the possible configurations of the d SOLVED: The compound [Ni(H2O)6]Cl2 is paramagnetic. Determine the oxidation number of nickel in this compound, the most likely geometry of the coordination around the nickel, and the possible configurations of the d](https://cdn.numerade.com/ask_previews/543ac424-a25c-42c6-88c8-ae8f1d1a07d2_large.jpg)
SOLVED: The compound [Ni(H2O)6]Cl2 is paramagnetic. Determine the oxidation number of nickel in this compound, the most likely geometry of the coordination around the nickel, and the possible configurations of the d
![IUCr) Crystal structure of a nickel compound comprising two nickel(II) complexes with different ligand environments: [Ni(tren)(H2O)2][Ni(H2O)6 ](SO4)2 IUCr) Crystal structure of a nickel compound comprising two nickel(II) complexes with different ligand environments: [Ni(tren)(H2O)2][Ni(H2O)6 ](SO4)2](https://journals.iucr.org/e/issues/2020/03/00/xi2016/xi2016scheme1.gif)
IUCr) Crystal structure of a nickel compound comprising two nickel(II) complexes with different ligand environments: [Ni(tren)(H2O)2][Ni(H2O)6 ](SO4)2
2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect Crystal structure and properties of the precursor [Ni(H2O)6](HTBA)2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0277538713008310-fx2.jpg)
Crystal structure and properties of the precursor [Ni(H2O)6](HTBA)2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect
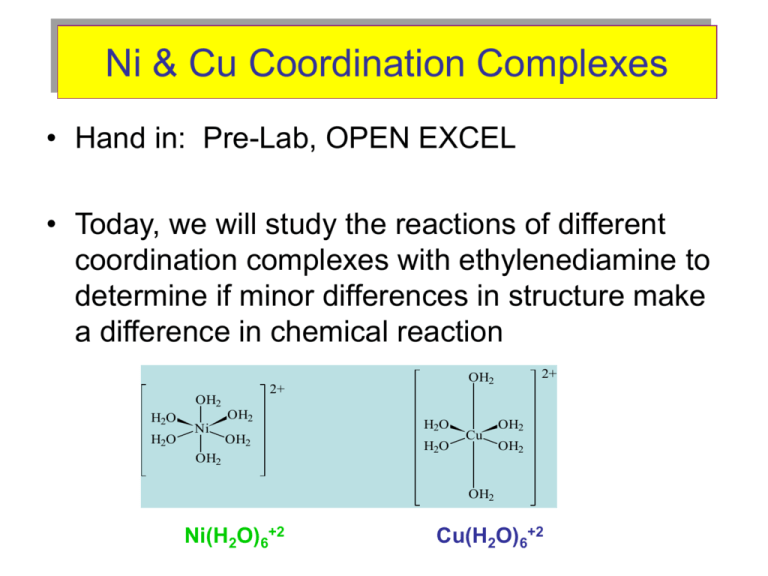
a. [Ni(H2O)6]^2+ (aq) is green in colour whereas [Ni(H2O)4 (en)^2+ (aq)is blue in colour , give reason in support of your answer . - Sarthaks eConnect | Largest Online Education Community
![Assertion A solution of `[Ni(H_(2)O)_(6)]^(2+)` is green but a solution of `[Ni(CN)_(4)]^(2+)` is - YouTube Assertion A solution of `[Ni(H_(2)O)_(6)]^(2+)` is green but a solution of `[Ni(CN)_(4)]^(2+)` is - YouTube](https://i.ytimg.com/vi/nIpwGgykAQU/mqdefault.jpg)

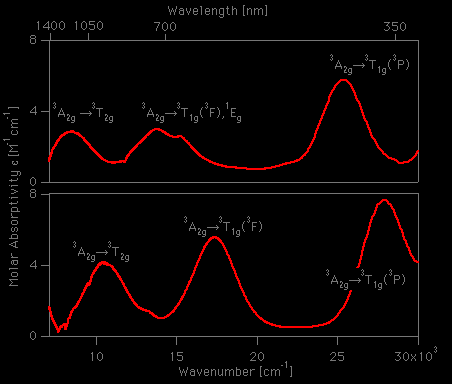
![a) Absorption spectrum of the [Ni(H2O)6]²⁺ complex in the spectral... | Download Scientific Diagram a) Absorption spectrum of the [Ni(H2O)6]²⁺ complex in the spectral... | Download Scientific Diagram](https://www.researchgate.net/profile/Petya-Petkova-7/publication/279288804/figure/fig1/AS:1132476902711296@1647014942438/a-Absorption-spectrum-of-the-NiH2O6-complex-in-the-spectral-region-395-795-nm_Q320.jpg)
![A solution of [Ni(H2O)6]^2 + is green but a solution of [Ni(CN)4]^2 - is colourless, Explain. A solution of [Ni(H2O)6]^2 + is green but a solution of [Ni(CN)4]^2 - is colourless, Explain.](https://i.ytimg.com/vi/HY7e_C1lrgo/maxresdefault.jpg)
2 with gaseous NH3; crystal growth via in-situ solvation - ScienceDirect Reaction of [Ni(H2O)6](NO3)2 with gaseous NH3; crystal growth via in-situ solvation - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0022024814007805-gr3.jpg)
![Write the IUPAC name of : [Ni (H2O)6 ]Cl2 Write the IUPAC name of : [Ni (H2O)6 ]Cl2](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/647499982_web.png)
![How [Co(H2O) 6] 2+ is having higher crystal field splitting energy than [Ni( H2O) 6] 2+? - Quora How [Co(H2O) 6] 2+ is having higher crystal field splitting energy than [Ni( H2O) 6] 2+? - Quora](https://qph.cf2.quoracdn.net/main-qimg-fa42f87b83e5b7668101dfb77e274e3c.webp)
2 and [Ni(D2O)6](ClO4)2 at Various Temperatures | Semantic Scholar FIR Spectra of [Ni(H2O)6](ClO4)2 and [Ni(D2O)6](ClO4)2 at Various Temperatures | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/ecaf6b0fee69dc0a5060cdb8d7e5d0c7d395a06e/4-Table1-1.png)

![The hybridisation in the metal ion of [ni(h2o)6]2 is - Brainly.in The hybridisation in the metal ion of [ni(h2o)6]2 is - Brainly.in](https://hi-static.z-dn.net/files/dbd/e83bcacc4342adef4784a0fdc1abce28.jpg)
![In [Ni(H(2)O)(6)]^(2+) , the hybridisation of central metal ion is : In [Ni(H(2)O)(6)]^(2+) , the hybridisation of central metal ion is :](https://d10lpgp6xz60nq.cloudfront.net/ss/web/875992.jpg)
![a) Absorption spectrum of the [Ni(H2O)6]²⁺ complex in the spectral... | Download Scientific Diagram a) Absorption spectrum of the [Ni(H2O)6]²⁺ complex in the spectral... | Download Scientific Diagram](https://www.researchgate.net/publication/279288804/figure/fig1/AS:1132476902711296@1647014942438/a-Absorption-spectrum-of-the-NiH2O6-complex-in-the-spectral-region-395-795-nm.jpg)
![A solution of [Ni(H2O)6]^2 + is green but a solution of [Ni(CN)4]^2 - is colourless, Explain. A solution of [Ni(H2O)6]^2 + is green but a solution of [Ni(CN)4]^2 - is colourless, Explain.](https://haygot.s3.amazonaws.com/questions/1955044_1567628_ans_f710a7dfc21d4ea7a5d920b41112c727.jpg)

![Solved (a) The Molecular Orbital Diagram for [Ni(H2O)6]2+ is | Chegg.com Solved (a) The Molecular Orbital Diagram for [Ni(H2O)6]2+ is | Chegg.com](https://media.cheggcdn.com/study/284/28408777-e2bb-4645-8742-fab9e76a7424/image)