Table 2 from Synthesis of Me Doped Mg(OH)2 Materials for Thermochemical Heat Storage | Semantic Scholar

Find out the solubility of Ni(OH)_2 in 0.1 MNaOH. Given that the ionic product of Ni(OH)_2 is 2 ×... - YouTube

Figure 4 from Preparation and Characterization of Ni ( OH ) 2 and NiOMesoporous Nanosheets | Semantic Scholar

Enhancing Hydrogen Evolution Activity in Water Splitting by Tailoring Li+-Ni (OH)2-Pt Interfaces | Science

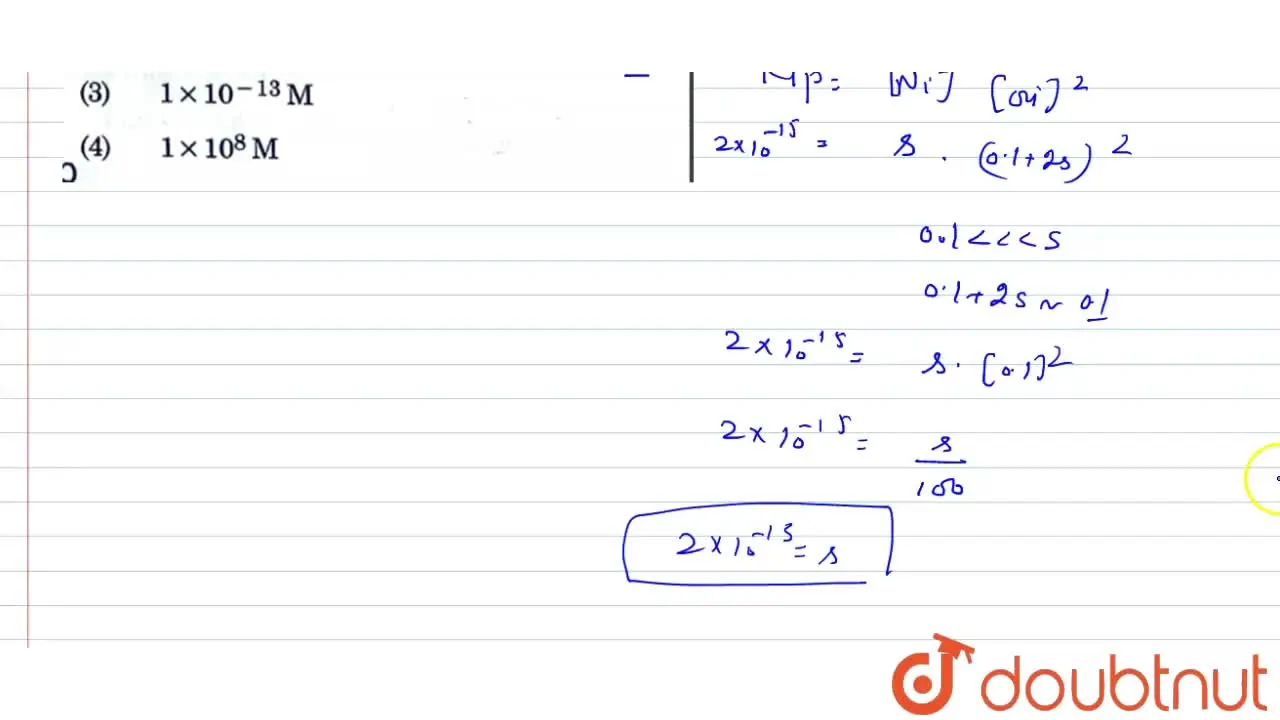
The solubility product of `Ni(OH)_(2)` is `2.0xx10^(-15)`. The molar solubility of `Ni(OH)_(2)` ... - YouTube

Strongly Coupled Ni/Ni(OH)2 Hybrid Nanocomposites as Highly Active Bifunctional Electrocatalysts for Overall Water Splitting | ACS Sustainable Chemistry & Engineering

Chapter 4: Aqueous Reactions Solution: Solvent: substance present in the larger amount Solute: substance(s) dissolved in solvent, generally present in. - ppt download
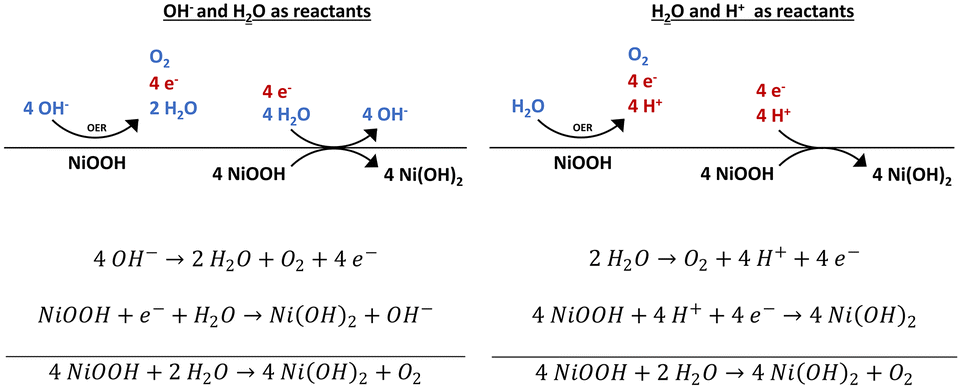
Stability and decomposition pathways of the NiOOH OER active phase of NiO x electrocatalysts at open circuit potential traced by ex situ and in situ s ... - Catalysis Science & Technology (

Ionic product of Ni{(OH)}_{2} is 2.0times {10}^{-15}. Molar solubility of Ni {(OH)}_{2} in 0.10M NaOH will be ______.1.0times {10}^{-13}M4.0times {10}^{-13}M8.0times {10}^{-13}M2.0times {10}^{-13}M

Catalytic Surface Specificity of Ni(OH)2‐Decorated Pt Nanocubes for the Hydrogen Evolution Reaction in an Alkaline Electrolyte - Hong - 2019 - ChemSusChem - Wiley Online Library
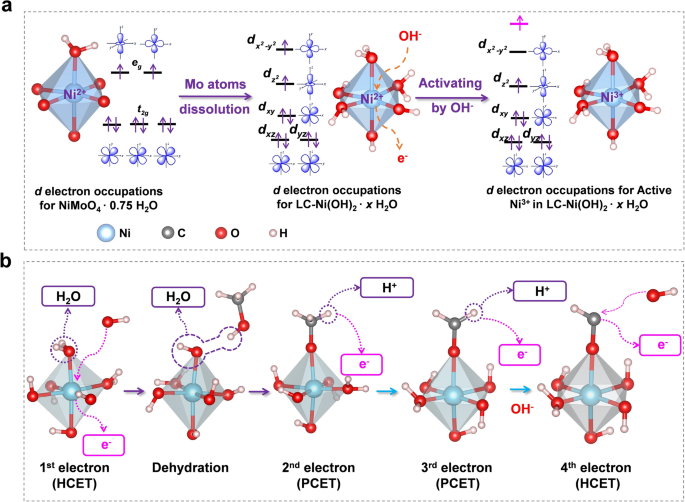
Coordination Effect-Promoted Durable Ni(OH)2 for Energy-Saving Hydrogen Evolution from Water/Methanol Co-Electrocatalysis | Nano-Micro Letters

Pt and Pt–Ni(OH)2 Electrodes for the Hydrogen Evolution Reaction in Alkaline Electrolytes and Their Nanoscaled Electrocatalysts - Ruqia - 2018 - ChemSusChem - Wiley Online Library

Interfacial electron rearrangement: Ni activated Ni(OH)2 for efficient hydrogen evolution - ScienceDirect

SOLVED: The pH of a saturated solution of nickel hydroxide, Ni(OH)2, is 8.83. Calculate the Ksp for nickel hydroxide. (Show all relevant chemical reactions and work.)

Ksp of a salt Ni(OH)2 is 2 x 10^-15 then, molar solubility of Ni(OH)2 in 0.01M NaOH is? - EduRev NEET Question